Fire Classes and Their Extinguishing Agents
Actualizado a fecha: 15 October, 2020
To extinguish a fire, we first need to know the different mechanisms that could have started it. Only then, can we find the most effective way to extinguish it according to its Fire Class.
For a fire to start, four important elements are required:
1.- A combustible material, which needs to be in the gas phase. It can be confusing: for instance, we think that solid wood burns when, in fact, what causes the first flame to appear is not the wood itself but the vapour coming out of it at a certain temperature.
2.- An Oxidising Agent. It is, most of the time, the oxygen present in the air. Without that element it is impossible to start a fire. The flame comes from the mix of an Oxidising Agent and a Combustible, both in the gas phase.
3.- An Activation Temperature. Part of the combustible material will start producing vapours, which mixed with the oxygen, will create the first flame; however, in order to get to that point, a certain temperature needs to be reached. That temperature is specific to each material and no fire can be started below it.
4.- A chain reaction. Once the first ignition appears, a chain reaction that liberates more vapour from the combustible material will be needed, as well as more oxygen, in order to keep the fire burning. This chain reaction can be linear, like a burning wick, or exponential, when a simple match turns into a large fire.
To successfully put out a fire, we need to get rid of at least one of these four elements. We will see how further down.
Fire Classes
According to the UNE EN 2-1994 /A1 norm, there are 5 Fire Classes depending on the nature of combustible material:
 Class A:
Class A:
Fires with a Solid combustible material, usually organic and which combustion will produce embers.
Examples: Wood, Paper, Cardboard, Carbon, Straw, Plastic, Rubber, etc.
A concrete example of this class of fires are the ones happening at home with wooden furniture, curtains, lots of plastic items, etc. The activation temperature in this case needs to be high for the vapours to starts emanating from the material and create the first flame.

Fires with a liquid combustible material.
Examples: Gasoline, Oil, Alcohol, etc.
During the liquid phase, there is always a small evaporation of those substances in the air and the activation temperature is lower. Special attention is required to avoid starting a chain reaction, like someone smoking at a gas station.
Fires with a combustible material in the gas phase at room temperature.
Examples: Natural Gas, Butane, Propane, Hydrogen, Acetylene, Propylene, etc.
Those fires are very present, in a controlled manner, in our daily life. The kitchen gas or gas boilers are the most common examples. Their activation temperature is the lowest of all and a single spark is enough to initiate the chain reaction at almost any temperature.

Fires with a metallic combustible material.
Examples: Aluminium Powder, Magnesium, Sodium, Potassium.
This Class of fires usually only happens in specific industries that work with those combustible metals. Those companies have strict security protocols and the few fires are only due to a lack of knowledge or negligence.

Fires with combustible materials derived from ingredients used in the kitchen.
Examples: vegetable and animal fats.
These fires could belong to the class B because of the liquid state of their combustible materials, but the vegetable or animal origins of said materials and their frequent use at very high temperatures makes them deserving of a category of their own.
Although some countries have a sixth Fire Class named “electrical fire”, this class does not exist in Europe. The reason is that electricity is at the origin of the fire and not the nature of the combustible material. But it is important to know it exists and always try to turn it off. If we can’t turn it off, we need to choose an adequate fire extinguisher with different levels of voltage.
Extinguishing Agents.
They can be products or just simple actions that help us put out the fire. In order to do so, we need to eliminate one or more of the four elements we talked about at the beginning of this article.
- Remove or divide the combustible material: it may seem the most elementary thing to do, but we sometimes forget it when we are dealing with a fire. When we find a fire, we need to identify the combustible elements surrounding it. Taking them away from the flames will break the chain reaction. The fire will not have any material left to burn and it will simply stop by itself. This is used to create firewalls in forests, dividing it in different zones. That way the fire cannot spread.
- Suffocate the fire by taking the oxidising agent away from it, basically leaving it without oxygen. This will make the flames automatically disappear. A common example is a flaming frying pan that we simply extinguish by covering with a Lid. No matter how much of the combustible material there is in the frying pan, it will not be burning without oxygen. Another example are the industrial kitchens or computer rooms equipped with a device that spreads gas all over the room, removing the oxygen.
- Cool the fire down: if we are able to make the temperature drop, we break the chain reaction. The combustible material is unable to reach its activation temperature and cannot maintain the combustion. Water, for example, attack the fire in two ways: it cools the fire down and, when transforming into vapour, chases the oxygen away and suffocates the fire.
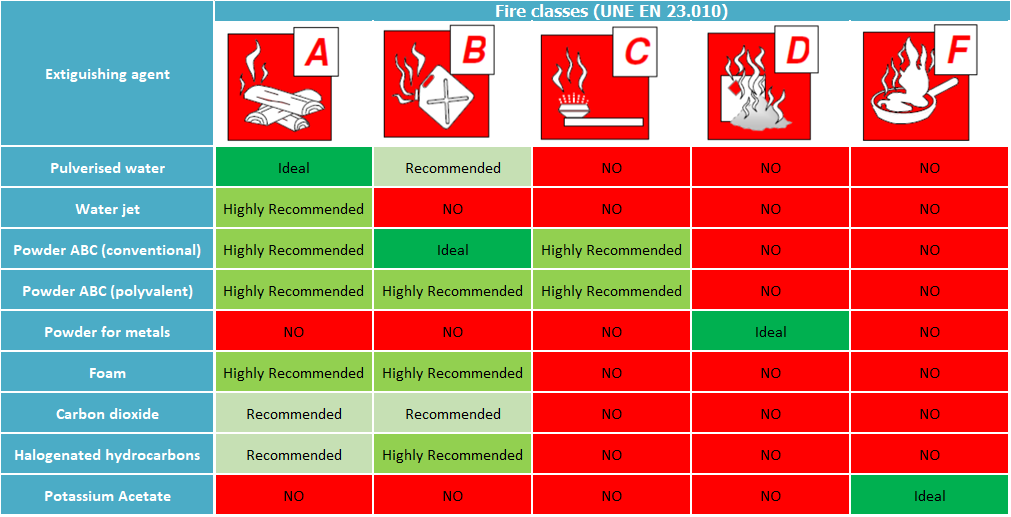
- Using fire extinguishers: The table below shows the principal types of extinguishers and their relevance according to the different Fire Classes: